By Eric Stann


May 20, 2025
Contact: Eric Stann, StannE@missouri.edu
Photo by Eric Stann
Imagine drawing on something as delicate as a living cell — without damaging it. Researchers at the University of Missouri have made this groundbreaking discovery using an unexpected combination of tools: frozen ethanol, electron beams and purple-tinted microbes.
By advancing a method called ice lithography, the team was able to etch incredibly small, detailed patterns directly onto fragile biological surfaces.
While traditional lithography is commonly used to make tiny circuits and other electronic parts for phones and computers, it relies on a liquid process that can easily harm delicate materials, including carbon nanotubes and biological membranes.
That’s where Mizzou’s ice-based approach stands out. By using a layer of frozen ethanol instead of liquid, they’ve created a gentler, more precise way to work with materials once considered too fragile to handle.
“Instead of using a traditional lithography process, which can be too harsh on delicate biological materials, our technique applies a thin layer of ice to protect the material’s surface while the pattern is made,” Gavin King, a professor of physics and study co-author, said. “That frozen layer helps keep everything stable during the process and makes it possible for us to work with delicate biological materials that would normally be damaged substantially.”
Mizzou has one of only three labs in the world — and the only one in North America — using this ice lithography method. What sets the work apart is the use of ethanol ice, which protects delicate biological materials where regular water ice would cause damage.
To test their new ethanol-ice-based method, researchers used Halobacterium salinarum, a tiny microorganism that makes a purple protein capable of capturing sunlight and turning it into energy — akin to nature’s version of a solar panel. Well known in biology since the 1970s, this microbe’s ability to efficiently convert light into energy makes it a promising candidate for developing new kinds of power sources.
While Mizzou’s discovery is proof of concept, the team is excited about its future potential, including the possibility of using these delicate purple membranes to create solar panels.
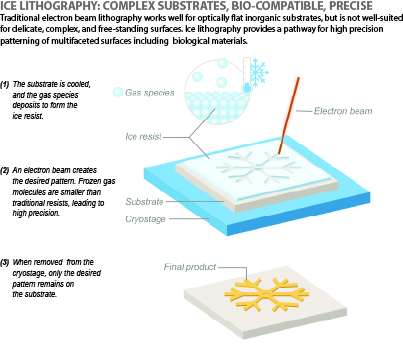
How it works
Here’s how the ice lithography method works.
First, researchers place the biological membrane on a cold surface inside a scanning electron microscope. The temperature is lowered to extremely cold levels, below -150°C. Then, when they add ethanol vapor, it instantly freezes into ethanol ice and forms a thin, smooth layer over the membrane.
Next, a focused beam of electrons draws tiny patterns in the frozen layer. Once completed, the surface is gently warmed. The parts of the ice that weren’t hit by the beam are sublimed away, while the pattern — now a solid material — is left behind.
“The patterns we’re making are smaller than 100 nanometers wide, and more than 1,000 times thinner than a strand of human hair,” Dylan Chiaro, graduate student and lead author of the study, said. “It's a major step toward working with some of biology’s most delicate components.”
A collaborative effort
This finding from researchers at Mizzou’s College of Arts and Science brings together the fields of biology, chemistry, physics and space science, and could transform how scientists work with the tiniest building blocks of life — molecules, proteins and atoms.
Suchi Guha, a professor of physics and study co-author, helped identify the structure of the resulting material. Using a high-sensitivity tool that examines how light interacts with molecules, called surface-enhanced Raman scattering, her lab discovered that the solid material behaves similarly to carbon fiber.
After the process was completed, the purple membrane was nearly unchanged — only losing less than one nanometer in thickness. This proves that researchers can use this process to create patterns directly on fragile biological materials without damaging them — a challenge that has perplexed scientists.
Bernadette Broderick, an assistant professor of chemistry and study co-author, helped discover the presence of ketene, a short-lived chemical that forms during the electron beam process. King believes this discovery by Broderick’s lab, which specializes in astrochemistry, can help explain how the ethanol ice transforms into a stable, solid material — a critical step in understanding the chemistry and physics behind the method.
“Each lab contributed a different piece of the puzzle,” King said. “This kind of interdisciplinary teamwork is what really made the discovery possible.”
“Precise fabrication of graphite-like material directly on a biological membrane enabled by ethanol ice resist,” was published in Nano Letters. Co-authors are Travis Hager, Kyle Renshaw, Bailey Moore and Arash Ghobadi at Mizzou; and Rubaiyet Haque and Anpan Han at the Technical University of Denmark.



