Published on Show Me Mizzou Sept. 17, 2025
Story by Chris Blose, MA ’03
Photos by Abbie Lankitus
When patients with prostate cancer first received a dose of Pluvicto at MU Health Care in 2023, they weren’t just getting one of the latest targeted cancer therapeutics. They were benefiting from decades of work performed by researchers, clinicians and technicians across the University of Missouri’s campus who made lutetium-177 (Lu-177) — the radioisotope in Pluvicto — a therapeutic reality. Lu-177 is a microscopic isotope on a monumental journey, from its production at the MU Research Reactor (MURR) to labs and treatment sites across campus, around the country and beyond. From its creation in the reactor to a patient’s bedside within about a week, Lu-177 has already changed lives.
At the Reactor: Birth of an Isotope

Power Core
As of 2025, MURR is the sole U.S. producer of Lu-177. Its roots with the isotope run much deeper: Mizzou researchers were among the first to identify its clinical potential in the early 2000s.
Since 2017, MURR has produced Lu-177 for:
- Academic researchers in the discovery phase, on campus and beyond
- Companion animal studies at Mizzou’s College of Veterinary Medicine
- Pharmaceutical partners developing therapies
- Clinical trials from preclinical through phase 3
Lu-177 is used in two FDA-approved targeted therapies available at MU Health Care and Ellis Fischel Cancer Center: Lutathera (2018) for neuroendocrine tumors and Pluvicto (2022) for metastatic prostate cancer. Both exist thanks to Mizzou’s work.

Molecular Promise
Carolyn Anderson has been at Mizzou since 2020, with an office across the street from MURR. But her connections to Lu-177 go back decades. “Lutetium-177 is a fascinating story, and I get to be a part of it,” she says.
When Anderson — the Simón-Ellebracht Professor of Chemistry and associate director of Ellis Fischel Cancer Center — was at Washington University in St. Louis in the early 2000s, a colleague at a medical development company flagged Lu-177 as an intriguing therapeutic target. That colleague soon learned MURR was already producing and studying the isotope for the same reason.
Anderson’s lab began receiving shipments of Lu-177 from Rotterdam, where her team performed the dosing studies that would eventually lead to Lutathera. “It is a really interesting isotope for a researcher,” she says. “It’s very easy to produce, and it has a perfect half-life. It also has the perfect beta-minus energy, which is something we look for.”
Now at Mizzou, Anderson is one of several researchers analyzing the potential of theranostics such as Lu-177 — treatments that combine therapeutics and diagnostics. Although it is primarily used in therapy, Lu-177 also has imaging capabilities for diagnosis.
Preparation and Targeting

From Molecule to Medicine
The half-life of Lu-177 is 6.6 days, so production, delivery and research all happen within about a week. At Ellis Fischel Cancer Center, those steps culminate in innovation trials and approved therapies.
MURR uses a high-flux neutron reactor to turn ytterbium-177 (Yb-177) into Lu-177 in a few hours via radioactive decay. The Lu-177 is separated from the Yb-177 and prepared for delivery to:
Academic labs, where Mizzou researchers are advancing cancer treatments, with Anderson’s team at the Life Sciences Center exploring new treatments and Jeffery Bryan leading early companion animal studies on Lu-177 in the 2000s.
Clinical trial centers, which can range from early phase trials to post-approval trials to expand treatment options. For example, Gregory Biedermann, a radiation oncologist, helped lead “innovation track” trials for Pluvicto at Ellis Fischel in 2023.
Pharmaceutical partners, for development, clinical testing and eventually commercialization and distribution to medical centers. In some cases, those partners are co-located on campus at a place such as MURR’s incubator space.
Medical centers, including MU Health Care and Ellis Fischel Cancer Center, where Lutathera and Pluvicto — both FDA-approved targeted cancer therapies that use Lu-177 — are available. Together, they bring cutting-edge therapies from the lab to patients.
On Target
Theranostics is a field that combines therapy and diagnostics. Its treatments are built to attack cancer cells while sparing healthy ones, using two parts: a radioisotope and a targeting molecule that steers it to the right place.
Lu-177 works well because its 6.6-day half-life allows time for use, its beta-minus decay is effective in treatment, and MURR can produce it at scale.
In Lutathera, Lu-177 is paired with dotatate, a small protein that latches onto receptors common in certain tumors, to treat neuroendocrine cancers. In Pluvicto, it’s paired with PSMA-617, which seeks out markers found on prostate cancer cells, to treat prostate cancer.
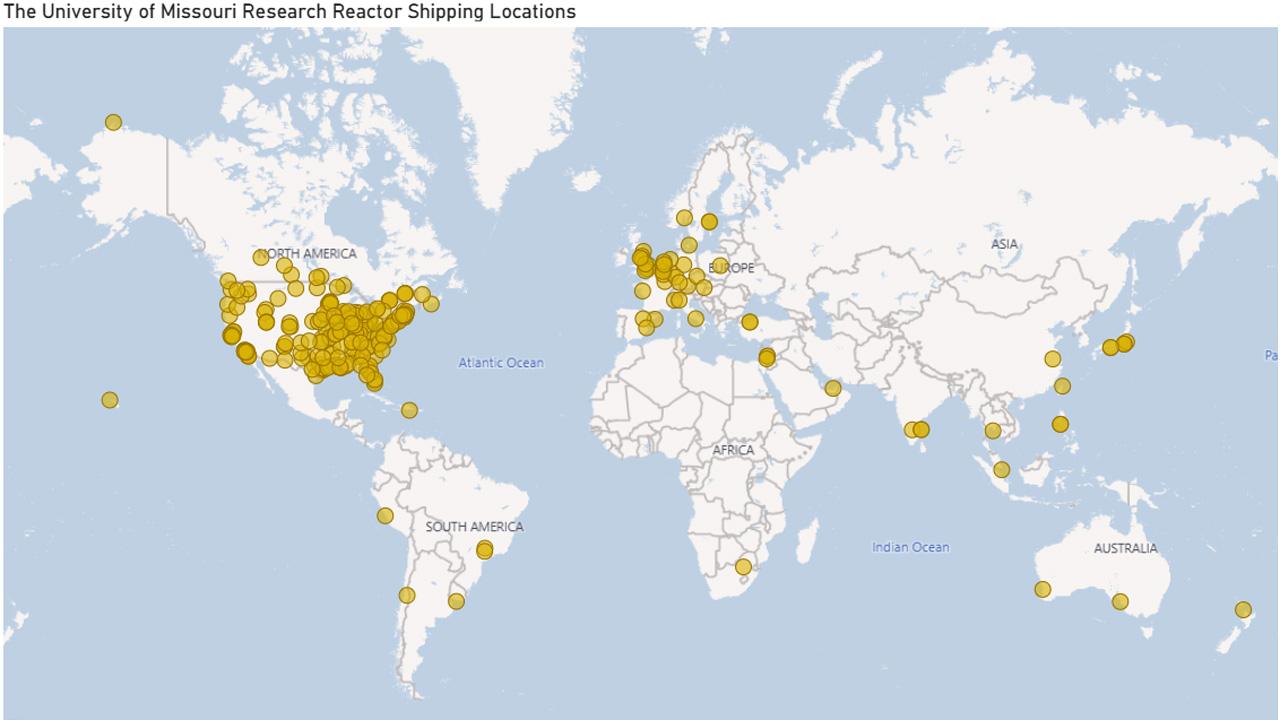
Distribution for Discovery
MURR isotopes reach Missouri, the nation and the world.
A Select History of Lu-177
- 1907: Lutetium discovered by Georges Urbain, Carl Auer von Welsbach and Charles James
- Early 2000s: MURR researchers and others identify the therapeutic potential of Lu-177
- 2001: Carolyn Anderson conducts toxicity studies with dotatate
- 2000s–2010s: Studies confirm safety and efficacy, including work at Mizzou’s College of Veterinary Medicine
- 2017: MURR begins large-scale production; Lutathera approved in Europe
- 2018: Lutathera approved by FDA and offered at Ellis Fischel Cancer Center
- 2022: Pluvicto approved in the U.S. and Europe
- 2023: Pluvicto innovation trials held at Ellis Fischel
- 2025: More than 50 trials under way; MURR remains sole U.S. producer
Patient Treatment and What’s Next

Nuclear Medicine’s Future
Demand and Supply
More than 50 trials are under way with Lu-177 and more than 2,000 with radioisotopes overall, which means rising demand for production.
Radioisotopes
MURR is the sole U.S. source of four cancer-targeting isotopes, including Lu-177. The proposed NextGen MURR would more than triple production capacity.
Talent
Mizzou’s infrastructure has attracted researchers such as Carolyn Anderson, and continued growth will require experts across chemistry, nuclear medicine, veterinary medicine and data science.
Partnerships
From Lutathera and Pluvicto to Therasphere, Quadramet and Ceretec, MU partners with industry to bring discoveries to patients. NextGen MURR would expand incubator and commercialization space.
Infrastructure
Meeting demand means updated labs, expanded facilities and a true bench-to-bedside model for nuclear medicine.
Lu-177 and Beyond
Many isotopes are in focus at Mizzou, including:
- Lutetium-177 (Lu-177): Beyond approved therapies, researchers are testing the isotope for melanoma, blood cancers and more.
- Terbium-161 (Tb-161): Produced at MURR and studied by Carolyn Anderson and Heather Hennkens, with strong cancer-fighting potential.
- Copper-64 and Copper-77: Cu-64 is used mainly in imaging, while Cu-77 shows promise for treatment.
- Yttrium-90 (Y-90): A microsphere-based therapy now in trials abroad aims to treat liver and colorectal cancers.
Expanding the Path
The journey of lutetium-177 at Mizzou doesn’t end with today’s therapies. It continues with new capacity for the future. The university has broken ground on two additions to the MU Research Reactor (MURR) that will expand the nation’s supply of cancer-fighting isotopes.
Together adding more than 29,000 square feet, the new wings will streamline operations and boost production of Lu-177. One will house three new production lines; the other will support further isotope manufacturing.
“MURR’s continued growth is essential to meeting the national demand for radioisotopes used in lifesaving, theranostics and cancer-fighting drugs,” University of Missouri President Mun Choi said. “This investment will allow us to help many more patients across the country and around the world.”
“As the only reactor in the world that runs 52 weeks per year, we are a reliable source of these much-needed radioisotopes for patients, medical providers and the pharmaceutical industry,” said Matt Sanford, executive director of MURR. “The demand continues to grow, and these new additions will provide more capability to meet the needs of the nation.”
To read more articles like this, become a Mizzou Alumni Association member and receive MIZZOU magazine in your mailbox. Click here to join.