Published on Show Me Mizzou April 30, 2024
Story by Chris Blose, MA ’04
Silence is one of the most troubling aspects of amyotrophic lateral sclerosis (ALS).
From the moment patients first notice symptoms of this neurodegenerative disease, also known as Lou Gehrig’s Disease, it takes an average of 10 months to get a diagnosis, according to research. Those 10 months of delay would be a challenge on their own. Over time, early symptoms such as muscle twitching and weakness and difficulty swallowing or speaking begin to devolve into devastating effects on the body’s motor functions. The disease is ultimately fatal.
But the delay from disease onset to diagnosis is much longer than those few months, says Smita Saxena, a University of Missouri professor and NextGen Precision Health researcher with expertise in cellular neurosciences.
“The disease process is quite insidious and silent,” Saxena says, “so we cannot say, ‘Oh, the disease started the day the patient came to the hospital. It could have started 20 years or 10 years beforehand.’”
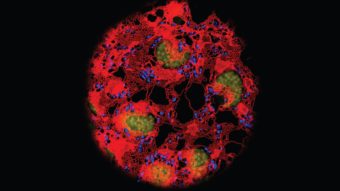
In that silent period before symptoms and diagnosis, the ALS disease process plays out in the body. A specific set of motor neurons may degenerate for years before the first physical signs show up, even while other brain cells function as normal. This phenomenon is called selective neuronal vulnerability, because only one set of neuronal cells is vulnerable to attack. It’s common across neurodegenerative diseases.
Saxena’s research is dedicated to understanding the phenomenon — and ultimately using that understanding not only to detect the disease earlier but also to develop better, more targeted therapies for a community of patients in need.
A pluripotent platform
As the crow flies, Bern, Switzerland, is 4,721 miles from Columbia, Missouri. The former is where Saxena built a high-profile career leading her own independent lab. The latter is where Saxena lives and works as of 2024 after being recruited to join NextGen Precision Health, a University of Missouri System-wide initiative staffed by researchers and clinicians.
She first came to the university in May 2023 as part of a series of seminars at the invitation of David Arnold, NextGen’s executive director. It wasn’t a recruitment visit, necessarily, but Saxena liked what she saw and heard.
Drawn to the facilities and equipment at the Roy Blunt NextGen Precision Health building, she was enthused by Arnold’s goal of growing a team of highly motivated neurosciences investigators. She also appreciated the close ties between basic and translational researchers and the doctors and other clinicians who work directly with patients.
“That is what we, the basic researchers, really look for is the liaison with the clinicians and the possibility to quickly move a target from bench to bedside,” Saxena says.
In turn, Arnold knew what Saxena could bring to the initiative. Arnold is a researcher and clinician in the field of ALS and other neurodegenerative diseases who joined NextGen in 2022. Since then, he has helped build a roster of investigators and doctors working on cardiovascular metabolic health, cancer, immunology, reproductive biology, bioengineering, medical imaging, autism and more. He has his own history of bench-to-bedside work, too, having been an investigator on a gene therapy for spinal muscular atrophy.
“I was recruited, I think, because of my background of being part of these big teams that really move new therapies to the clinic,” Arnold says. “And really, NextGen Precision Health, the initiative, is all the various parts of my life in one spot — with the goal to break down those barriers that sometimes separate healthcare and science.”
Arnold’s current priority is growth in neurosciences. Recruiting Saxena helps fill a major need for her fellow researchers.
“Probably the biggest gap until she got here is an approach called induced pluripotent stem cells,” Arnold says. “So that’s where you can take a skin cell, and you basically erase the genetic markers of it. You can turn it back into a stem cell, so then you can turn it into a neuron or a muscle cell or any cell like that.”
The value of pluripotent stem cells is hard to overstate. Its effectiveness is like taking a fully written story — potentially one with a bad ending — and turning it back into a blank page, ready to be rewritten however needed.
Researchers can use these flexible cells to model different stages of disease, to see how neurons respond to currently available treatments, and ultimately to work toward treatment to what works best for individual patients. Imagine being able to test a specific therapy on a specific patient, using their very own cells. It’s as precise as precision medicine can get.
An estimated 32,000 people in America live with the disease, according to the CDC, and those numbers are likely underreported. Yet even for a rare disease with a relatively small population, a one-size-fits-all treatment is unlikely to work for every patient.
“Eventually, what we are achieving is a precision medicine which is tailored where we have found, perhaps, molecules that are specifically working on those patients’ neurons,” Saxena says. “My biggest goal here in Missouri is to actually set up these model systems because they are not yet available here.”

Breaking through barriers
Saxena divides her research agenda into three parts, based on key questions. The answers have implications for ALS, but also possible applications for other patients, as well. (See “Beyond ALS” below.)
The first, and most basic question: When does the disease really start?
Saxena’s expertise at the cellular level comes into play here. She and her team examine the specific neurons that are affected by ALS, and how they behave and respond. “Our brain is plastic,” says Saxena, referring to the brain’s ability to adjust and adapt, “so the neurons have the capacity very early on to actually resist the onset of disease. And they do this by initiating adaptive or compensatory responses.”
Saxena’s working hypothesis is that once those adaptive responses fail, the disease process begins in earnest. With that in mind, future therapeutics might focus on helping neurons maintain their adaptive responses, and thus their ability to fight ALS.
However, doing so requires a strong understanding of where a patient is in the disease process, which leads to the second question: How can we determine disease stages?
Biomarkers offer one potential answer. Clinicians use these molecules to identify abnormal processes in the body, including disease. “Right now, we don’t have biomarkers that can predict and say, this patient is in stage one, stage two or stage three of the disease,” Saxena says. “So can we find those biomarkers which could enable us to predict where and how far the disease has progressed in this patient?”
Understanding and then staging the disease lead to the ultimate question: What are the best therapies?
Treatments for neurodegenerative diseases often work best when delivered directly to the brain. Therein lies the challenge. Drugs must be able to penetrate the blood-brain barrier, essentially a border that separates the circulatory system and the central nervous system.
“Very often an orally given drug is largely metabolized in the liver and then excreted out. So we work with molecules, like nanolipids, if we have a potential drug target. It’s a quite a complex chemical process, so we work together with biotech companies to do that for us. And then, once we have these nanolipids, they can easily cross the blood-brain barrier. They bring along your target molecule into the brain, and then they can be delivered to the right areas of the brain for therapy.”
For instance, Saxena previously collaborated on research showing that one particular protein, cerebral dopamine neurotrophic factor (CDNF), protects the neurons that are vulnerable in ALS. On top of that, CDNF can pass the brain-blood barrier. As a treatment, it’s promising enough that a multicountry collaboration is pushing it into human clinical trials for movement disorders like Parkinson’s disease and ALS.
Still, researchers and clinicians alike know that no one drug will solve everything for every patient. The work continues in all phases, from the most basic science to the translational work that takes a discovery from bench to bedside.
“I don’t believe that there will be one single magic bullet,” Saxena says. “This is going to be a combination of therapy. So, we need to find several different therapeutic targets that would work together.”
Beyond ALS
When Smita Saxena joined the NextGen Precision Health team this year, she brought her expertise in ALS with her. However, her work, and that of her colleagues, has applications beyond just one disease.
Saxena and David Arnold, executive director of NextGen, shared a couple of other priorities for the growing neurosciences team:
One is applying existing techniques or discoveries to other diseases, since principles such as selective vulnerability apply across the board for neurodegenerative diseases. For example, CDNF, a promising neuroprotective protein that Saxena helped identify as a potential drug target, has possible applications across various neurodegenerative diseases.
The team also focuses on autism now, thanks to a collaborative initiative between the university and the nonprofit Genetic Autism Alliance. The goal is to seek and develop targeted treatments for genetic forms of autism. Saxena’s induced pluripotent stem cells will be especially helpful in this search, because they can be used to test specific therapies on specific cells.
Arnold says Saxena’s cell platform should apply to diseases beyond the nervous system, so it’s a boon for the entirety of NextGen. “It really will have a ripple effect,” he says. “And that’s one requirement of NextGen Precision Health. You are required to collaborate. Team science is a prerequisite to be part of the team.”
To read more articles like this, become a Mizzou Alumni Association member and receive MIZZOU magazine in your mailbox. Click here to join.